Motoyuki Shiga and Wataru Shinoda
J. Chem. Phys. 123 134502 (2005).
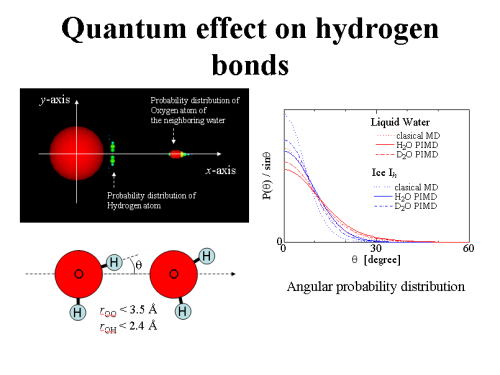
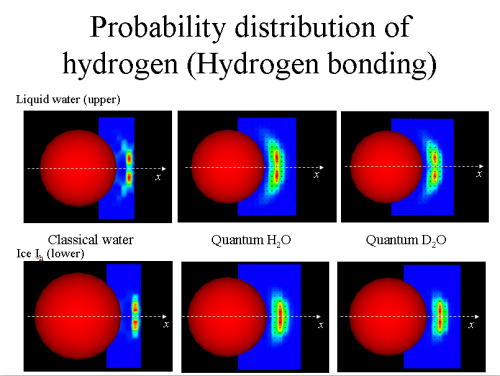
As an application of atomistic simulation methods to heat capacities, path integral molecular dynamics has been used to calculate the constant-volume heat capacities of light and heavy water in the gas, liquid, and solid phases. While the classical simulations based on conventional molecular dynamics has estimated the heat capacities too high, the quantum simulation based on path integral molecular dynamics has given reasonable results based on the simple point charge/flexible potential model. The calculated heat capacities (divided by the Boltzmann constant) in the quantum simulation are 3.1 in the vapor H2O at 300 K, 6.9 in the liquid H2O at 300 K, and 4.1 in the ice Ih H2O at 250 K, respectively, which are comparable to the experimental data 3.04, 8.9, and 4.1, respectively. The quantum simulation also reproduces the isotope effect. The heat capacity in the liquid D2O has been calculated to be 10% higher than that of H2O, while it is 13% higher in experiment. The results demonstrate that the path integral simulation is a promising approach to quantitatively evaluate the heat capacities for molecular systems, taking account of quantum mechanical vibrations as well as strongly anharmonic motions.


Return to HOME