DPPC DPhPC
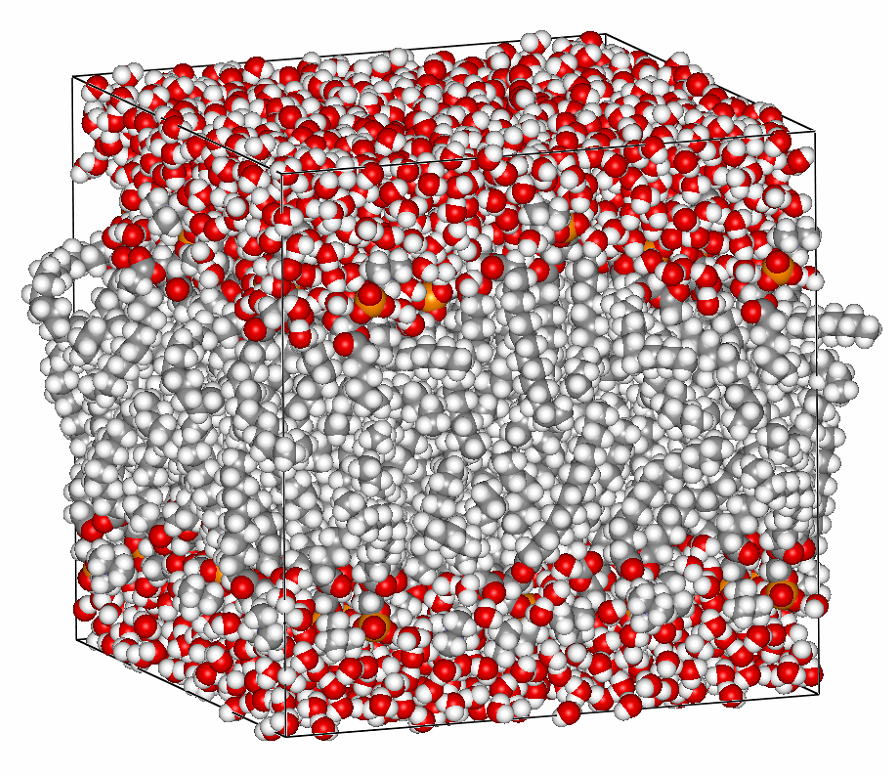
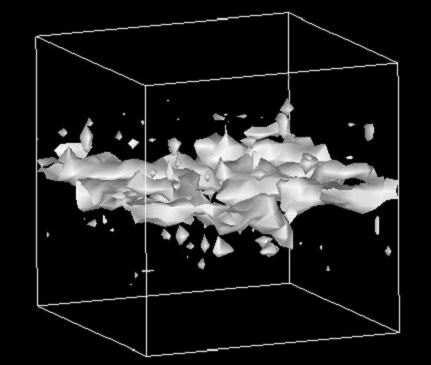
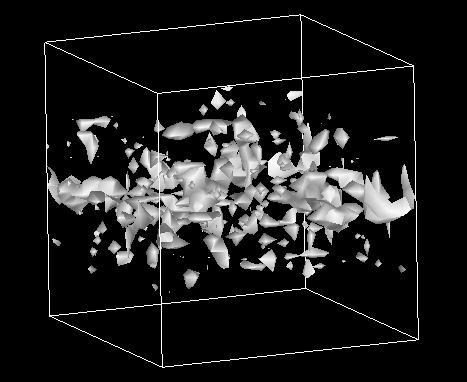
Cavity distribution in lipid bilayers
Wataru Shinoda, Masuhiro Mikami, Teruhiko Baba, and Masakatsu Hato
J. Phys. Chem. B 108, 9346-9356 (2004). (PDF is available from JPCB)
We studied the effects of chain branching on the water and nonionic (neutral) solute permeability of lipid bilayers in a molecular dynamics simulation comparing two bilayers: dipalmitoylphosphatidylcholine (DPPC) and diphytanoylphosphatidylcholine (DPhPC). The calculated free energy profiles of several neutral solute and water molecules across the lipid membranes showed that chain branching caused no significant changes in the solubility of these molecules inside the membrane core. However, an analysis of the cavity distribution in each of these bilayer systems demonstrated that the branch-chained DPhPC bilayer had, compared with the straight-chained DPPC bilayer, a relatively small and discrete free volume distribution in the hydrophobic part. This suggests that small penetrants have a lower rate of diffusion inside branch-chained lipid bilayers. Actually, water molecules showed lower local diffusion coefficients inside DPhPC membrane than inside DPPC membrane. The low penetrant mobility of the former must correlate with the slower dynamics of the branched DPhPC chains. Thus, we conclude that chain branching effects on the permeability are, as far as neutral small penetrants are concerned, attributable mainly to the reduction of chain dynamics. The effects of chain branching on proton permeability are also discussed in the context of the proton-wire hypothesis.



DPPC DPhPC
Cavity distribution in lipid bilayers