S. Mieda, A. Ikeda, Y. Shigeri, W. Shinoda
J. Phys. Chem. C 118, 12555-12561 (2014).
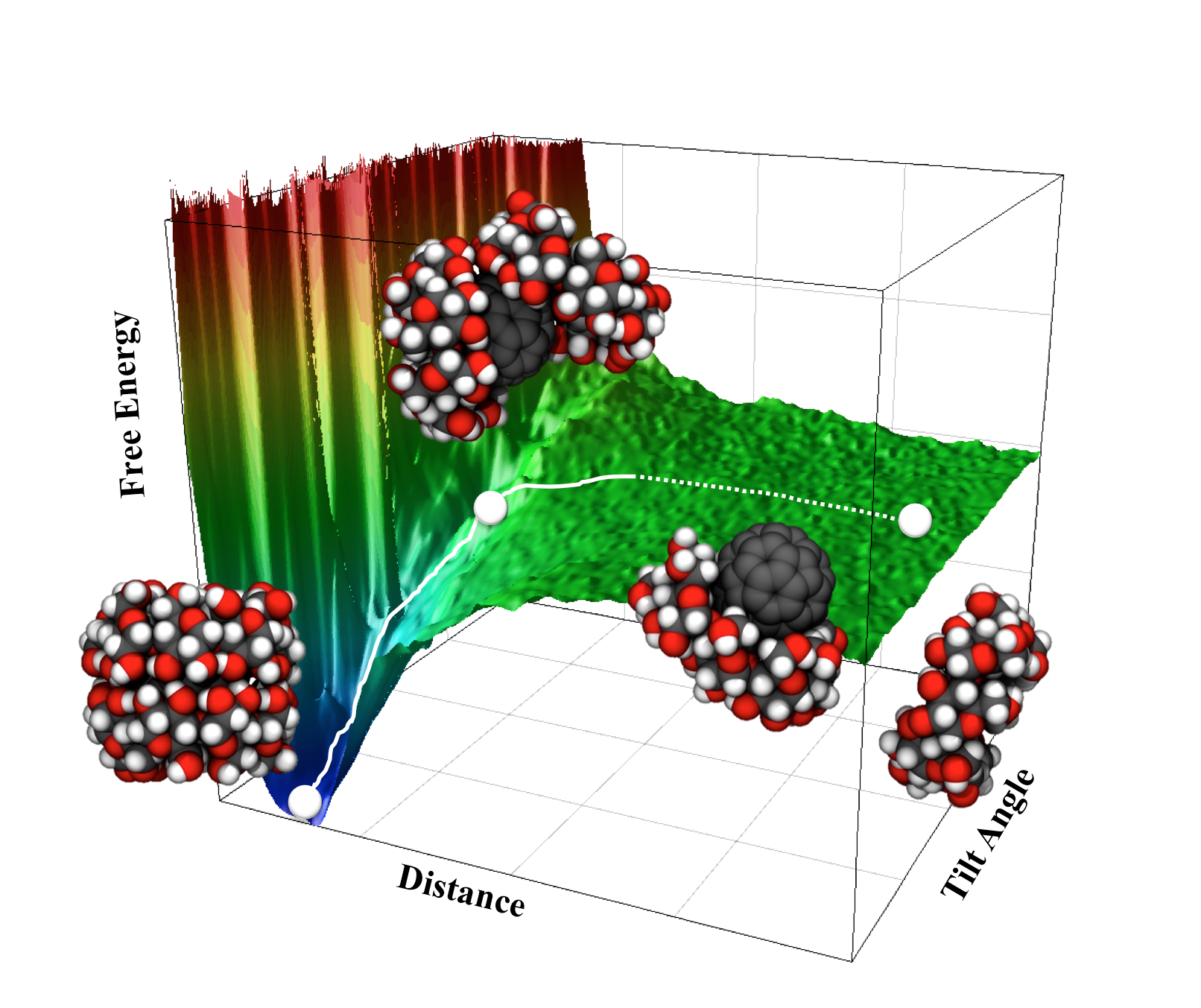
Using a supramolecular approach, water-soluble fullerenes were developed to increase the applicability of fullerenes in aqueous environments. γ-Cyclodextrin bicapped fullerene (C60¥γ-CD) complex was proposed as one of the strong candidates to show high solubility and stability in aqueous solution. In this work, a series of free energy simulations of C60¥γ-CD complex in both water and aqueous DMSO solution has been carried out to understand the effect of solvents on the thermodynamic stability of the complex. A high stability of the complex was observed in these solvents, although the stability was slightly reduced by the addition of DMSO to aqueous solution. The reduced stability in DMSO was illuminated by the change of complex structure, as well as solvation structure. The two-dimensional free energy map for the dissociation of the C60¥γ-CD complex elucidated a possible pathway for a dissociation of single γ-CD from the C60¥γ-CD complex. The information obtained on the molecular view point, might be useful to design a new stable water-soluble complex based on fullerene derivatives.
