Kotono Takeuchi, An-Tsung Kuo, Takeshi Hirai, Tatsuya Miyajima, Shingo Urata, Susumu Okazaki, and Wataru Shinoda,*
J. Phys. Chem. C, 123, 20628-20638 (2019).
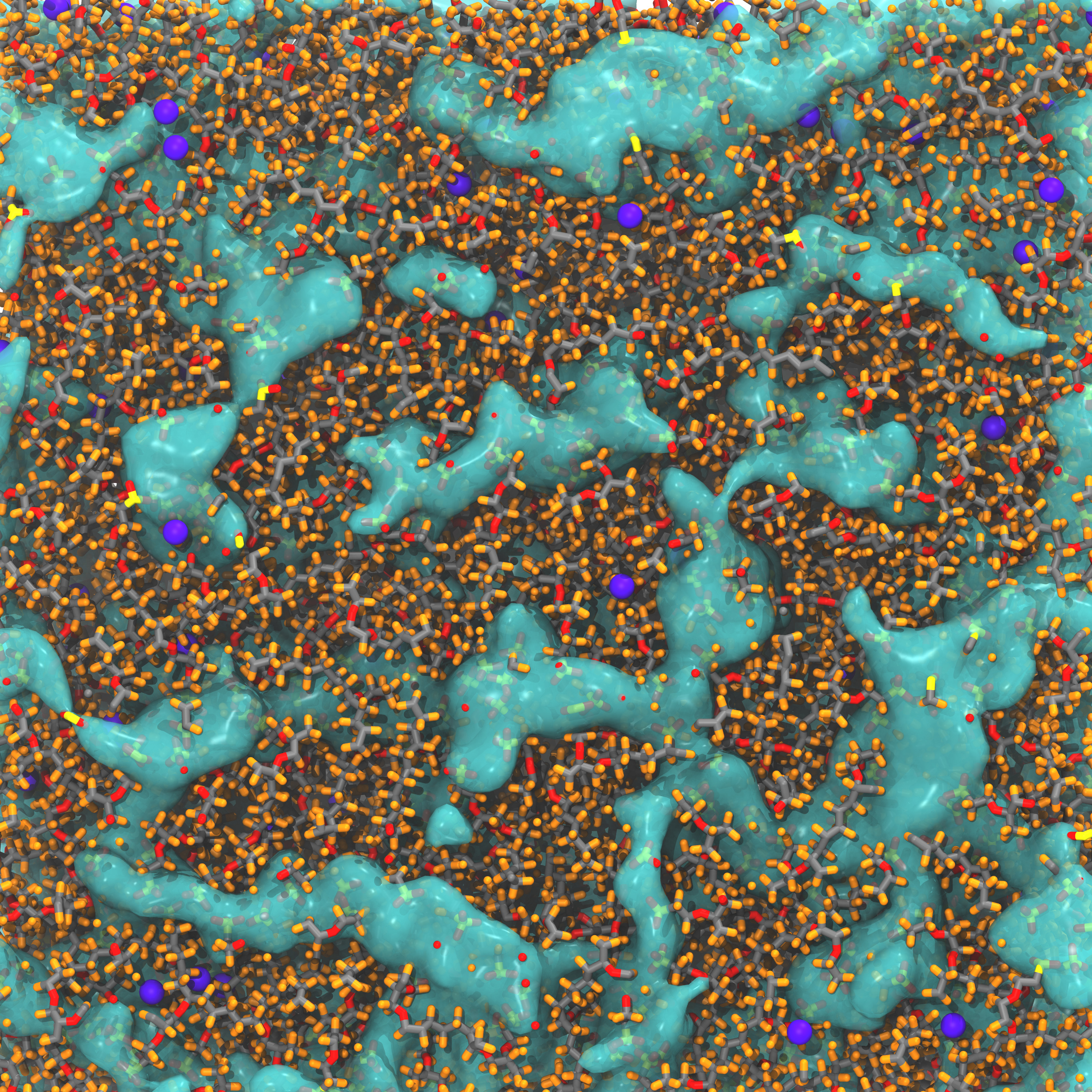
Gas permeation through proton exchange membranes affects a fuel cell's electrochemical performance. To understand the gas permeation mechanism, gas permeability and wide angle X-ray diffraction were measured for different equivalent weight (EW) perfluorosulfonic acid (PFSA) membranes at different water uptakes. A series of molecular dynamics (MD) simulations were also conducted. Through MD simulation, water sorption effects were found to result from an increased probability of dissolved H2 molecules being near the water phase, where a higher H2 diffusivity was observed. Furthermore, the local semi-crystalline structure of the PFSA polymer and the morphology of the water clusters in the membrane were found to affect H2 permeation in the high- and low-EW PFSA membranes, respectively. For the high-EW (EW > 909) membranes, the presence of the local crystalline structure decreased the void fraction in the structure and inhibits the H2 diffusion across the aligned polymer chains. This effectively reduced H2 solubility and diffusivity, thereby reducing H2 permeability. The low-EW membranes contained a lower number of PTFE regions and exhibited a highly tortuous network in the aqueous domain. The former contributed to a reduction in H2 solubility and the latter reduced the H2 diffusivity. Therefore, H2 permeability decreased with a decreased EW.
