Shuhei Ikeda, Seiji Tsuzuki, Taku Sudoh, Keisuke Shigenobu, Kazuhide Ueno*, Kaoru Dokko, Masayoshi Watanabe, and Wataru Shinoda*
J. Phys. Chem. C, 127 13837-13845 (2023).
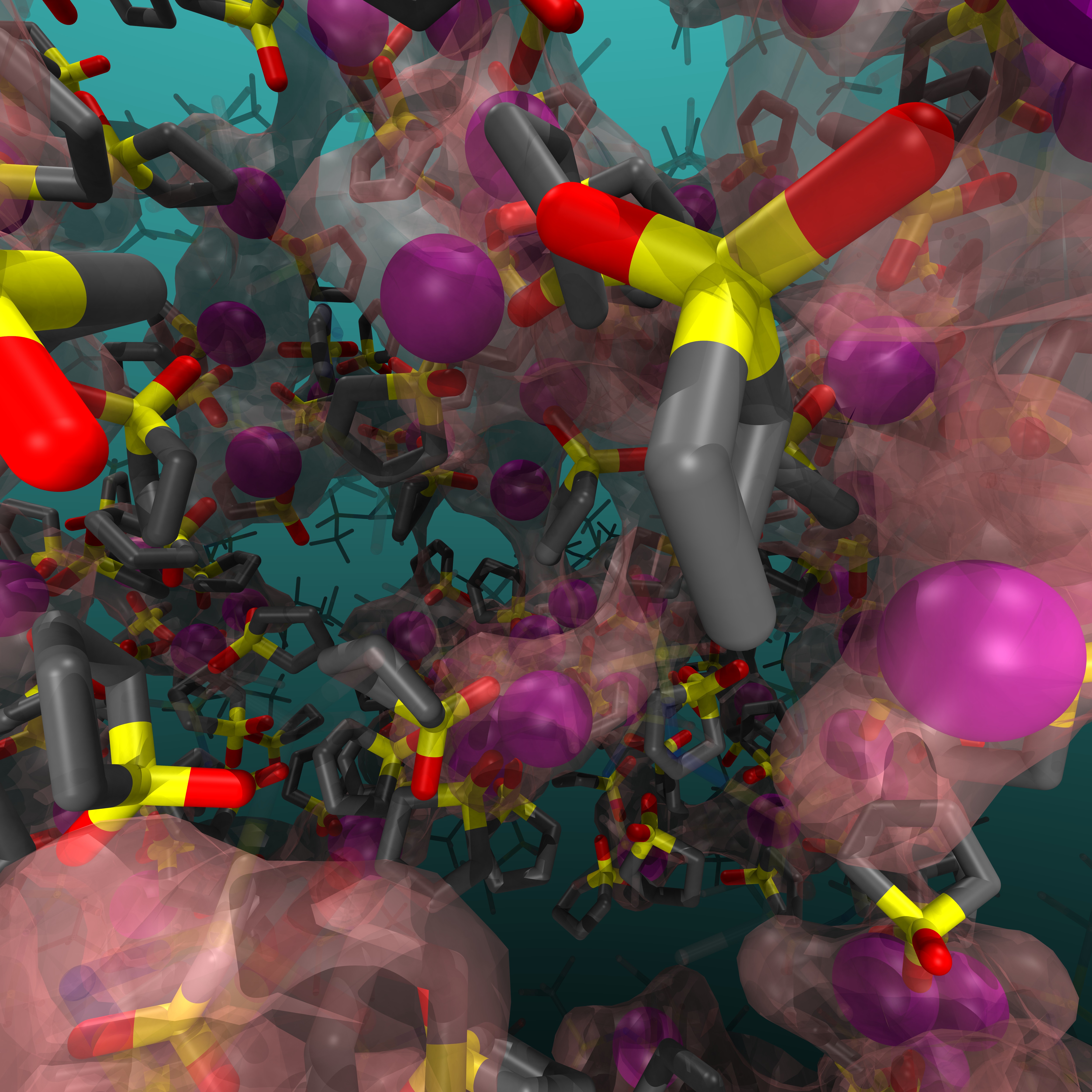
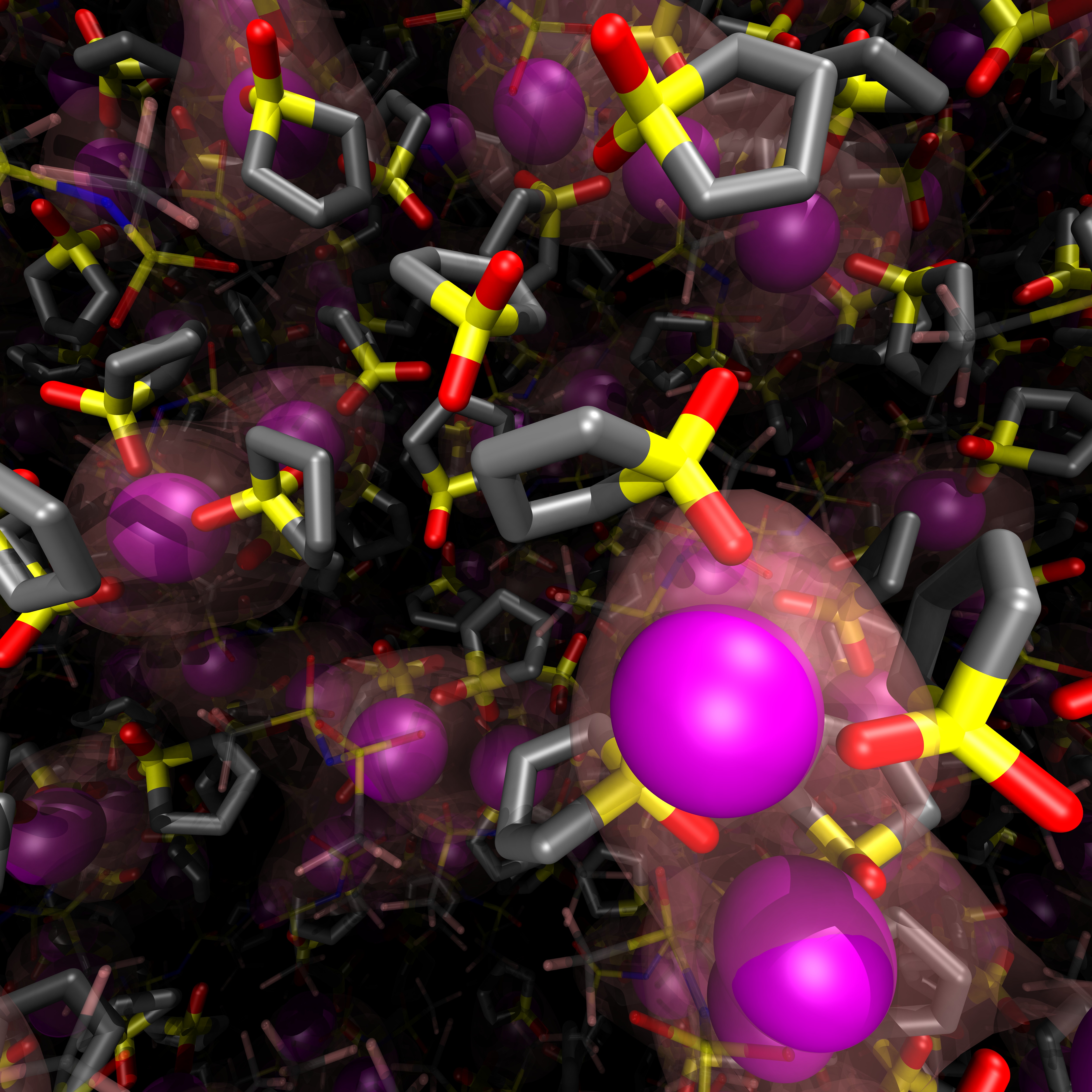
Here we report the use of molecular dynamics simulations with a polarizable force field to investigate Li-ion dynamics in sulfolane (SL)-based electrolytes. In SL-based highly concentrated electrolyte (HCE) (e.g., SL:Li = 2:1), Li displays faster translational motion than other components, which should be related to the structural and dynamical properties of SL. In HCE, a transient conduction network that penetrated the simulation system was always observed. Rapid (<1 ns) Li-ion hopping between adjacent coordination sites was observed throughout the network. Additionally, SLs rotated in the same timeframe without disrupting the conduction network. This rotation is believed to promote hopping diffusion in the network. This was followed by a rotational relaxation of the SL dipole axis around the non-polar cyclohydrocarbon segment of the SL (~3.3 ns), which involves a reorganization of the network structure and an enhancement of the translational motion of the coordinating Li-ions. The observed lifetime of Li-SL coordination was longer (>11 ns). Hence, it was concluded that the faster Li translational motion was obtained due to the faster rotational relaxation time of SL rather than the lifetime of Li-SL binding. The faster rotation of SL is related to its amphiphilic molecular structure with compact non-polar segments. Transport properties such as the Onsager transport coefficients, ionic conductivity, and transference number under anion-blocking conditions, were also analyzed to characterize the feature of the SL-based electrolyte.

