Keiko Shinoda, Wataru Shinoda, and Masuhiro Mikami
Phys. Chem. Chem. Phys. 9 643 (2007).
We have performed molecular dynamics simulations of a bilayer formed by the synthetic archaeal lipid, diphytanyl phosphatidylcholine, in NaCl electrolyte solution at four different concentrations (0-4 M) to investigate how structural and dynamical properties of the model archaeal membrane are changed due to the ionic strength in the solution. The archaeal lipid bilayer shows minor changes in their physical properties, indicating unusual high stability of the membrane against salt, though small reductions of molecular area and lateral diffusion of the lipid are detected at the highest electrolyte concentration of 4M. Sodium ions penetrate to the ether-rich region, where the ions are likely bound to the ether oxygen in the sn-1 chain rather than to that in the sn-2 chain. The observed salt bridges among two or three neighboring lipids accounts for the small reduction of the molecular area. The bound ions together with the counter (chloride) ions give rise to a diffusive electric double layer; as a result, the membrane dipole potential is slightly increased with increasing NaCl concentration.
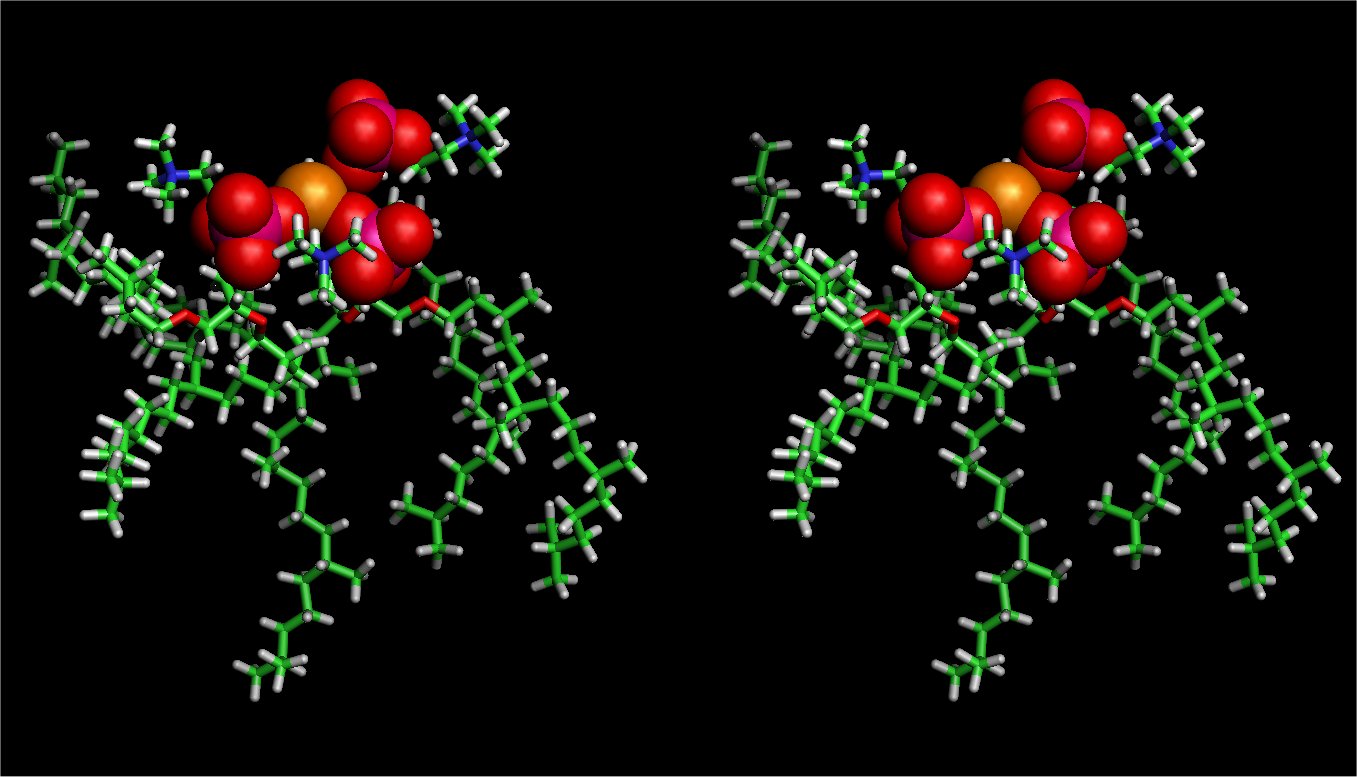
A snapshot of salt-bridge