Lipid Membranes/
Biological MembranesMolecular Simulation
Membrane Morphology
Lipid membranes change its morphology depending on their lipid components, thermodynamic and solution conditions such as temperature, pH, and salt concentration, and also by the addition of proteins, peptides, and nanoparticles interacting with the membranes. We are interested in the molecular process to cause the morphology changes of membranes. Biological membranes are not homogeneous, showing the heterogeneous distribution of lipid components not only bewteen inner and outer leaflets but also in the same leaflet. Many research works have been done on heterogeneous membrane structure in terms of biological functions. We have done a coarse-grained molecular modeling to investigate the membrane morphology including curved membranes such as vesicles using molecular dynamics simulation. In a mixed lipid vesicle, the free energy required for the vesicle deformation is reduced due to the lipid sorting (Pure Appl. Chem. 2014). We are working on the effect of additives (amphiphilic peptides, proteins, nanoparticles) on the free energy barrier for morphology changes.
 |
| Lipid distribution in spherical and deformed vesicles made of DMPC and DOPE mixture. (Pure Appl. Chem. 2014) |
Membrane Fusion
Membrane fusion is a fundamental biological process occurring in cell membranes. Therefore, the molecular mechanism of the fusion process has been widely investigated. The process should be affected by local lipid components, hydration, electrostatic interaction, proteins interplaying with membranes, and so on. We have developed a coarse-grained molecular model to treat large-scale phenomena such as membrane fusion involving vesicles. We have also proposed a free energy method to evaluate the free energy barrier along the stalk mechanism of the membrane fusion. Using these computational tools, we now try to understand what reduces or increases the free energy barrier of the membrane fusion process quantitatively.
 |
| Simulation of a vesicle interacting with a membrane (Ref: Science, 2008) |
We have developed a method to evaluate the free energy barrier of membrane fusion along the stalk mechanism using the guiding wall potential. The method is useful to characterize the free energy profile from the separated (apposed) membranes to stalk formation, and then, to fusion pore formation(Kawamoto & Shinoda, 2014). Using the method, effects of membrane curvature and lipid components on free energy barriers of the membrane fusion has been successfully evaluated using the SDK (recently renamed as SPICA) coarse-grained (CG) molecular model (Kawamoto,Klein,Shinoda, 2015).
 |
| Snapshots of the simulated systems during the course of free energy calculation of membrane fusion. (Ref. Kawamoto et al. J. Chem. Phys. 2015) |
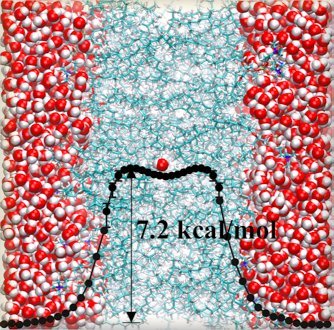
Permeability, Transportation of small molecules across lipid membranes
Permeability of small molecules through lipid membranes has been investigated by molecular dynamics simulations
on the basis of inhomogenous solubility-diffusion model. In this approach, free energy profile and local diffusion coefficient
of the small penetrant are needed to be calculated. Several free energy methods have been well
established for a small molecule. For example, free energy profile of a
single water to go across a lipid membrane is precisely calculated by a
combination of overlapping distribution method and cavity-based Widom insertion
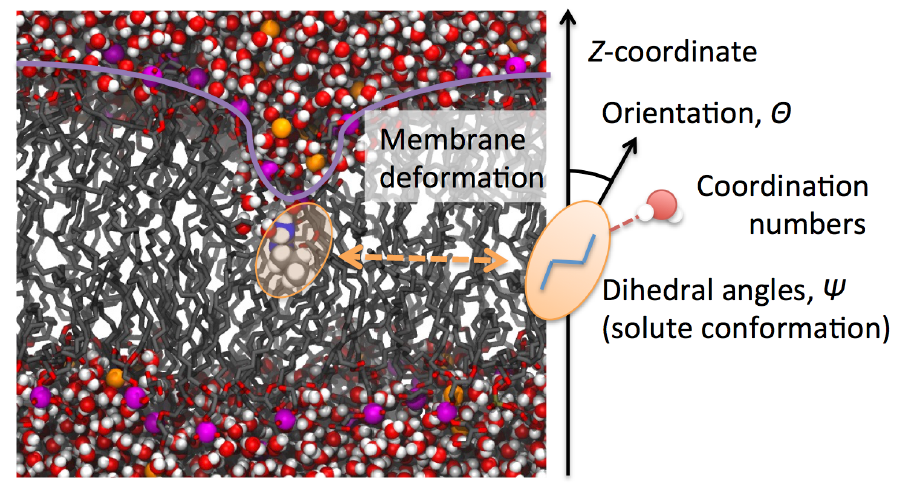
method. (Figure) However, it is still not straightforward to compute the
free energy profile for rather large molecules such as peptides and nanoparticles.
In this case, the permeation can be a collective dynamics involving multiple
molecules; namely, the choice of the reaction coordinate or collective
variables is nontrivial. We are trying to develop a simulation method to
understand such a complex molecular mechanism. We are also interested in
the kinetic factors to characterize the permeation process.
An overview of the recent progress on permeability assessment using molecular
dynamics simulations is given in our recent review paper (BBA-Biomembrane,
2016).
 |
 |
| Free energy profile of a water molecule to go across the DPPC membrane
(J. Comp. Chem. 2007) |
Possible slow variables required to characterize the permeation mechanism of a large complex molecule.(BBA-Biomembrane, 2016) |
Mechanical Properties of Membranes
Lipid membranes are conventionally described by a continuum theory based on the Helfrich Hamiltonian, in which the membrane is treated as an elastic sheet with zero thickness. The sucess of the continuum theory to characterize the membrane physics at the micrometer scale motivated us to compute the elastic constants from molecular dynamics simulations. By having a good estimate of elastic constants from MD, we can predict the mesoscopic elastic behavior of vesicles based on the molecular details. A comparison of MD and experimental results for the elastic properties provides a good examination for the molecular models, too. One of the examples in this kind of research activities is to develop a method of a free energy evaluation of a membrane as a function of imposed curvature. (Figure) Pressure profiles across the membranes are also useful to evaluate the elastic constants and spontaneous curvatures. Now the pressure profile calculation is also applicable to a spherical coordinate, which is useful for spherical vesicles.
 |
| Curved membranes supported by the guiding external potential (black circle).
Bending modulus is estimated by the relation between the membrane curvature
and the force required to support the membranes. (J. Chem. Phys. 2013) |
<<Back to the previous page








